Abstract
Background
The introduction of novel agents has contributed to improvements in survival in multiple myeloma (MM), and, based on phase 3 investigations, multiple novel regimens have been recently approved. Furthermore, clinical trials have demonstrated that the paradigm of continuous or extended therapy may offer improved outcomes vs shorter-term approaches. However, despite improvements in efficacy seen in the clinical trials setting, which is typically rigorously controlled, the same results are not always achieved in the real-world setting (effectiveness). This gap is associated on one hand with patient selection in clinical trials and on the other hand with toxicity burden, patient and physician motivation, different distribution of academic vs community centers, access issues, and other factors contributing to premature discontinuation of treatment regimens outside of clinical studies. Furthermore, there are inherent difficulties in obtaining and analyzing real-world data. Several methods can be used, including prospectively designed observational studies, retrospective chart reviews, and claims database analyses, although currently there are no standardized methods for claims-based outcomes research in MM. Thus, there is an important unmet need to understand effectiveness and outcomes seen in the real world. We conducted a systematic literature review of real-world, non-clinical-trial data in RRMM and reviewed findings in the context of recently reported phase 2/3 trial results.
Methods
We conducted a search of PubMed for publications containing real-world RRMM data published in the past 10 years using a range of relevant search terms including 'real-world', 'EMR', 'community', and 'registry' (N=934). Abstracts from the past 3 annual meetings of ASH, ASCO, and EHA, and from the past 2 IMW meetings were similarly reviewed (N=93). Relevant publications (n=10) and abstracts (n=37) were manually selected. For the purposes of the present analyses, data were extracted from reports of real-world data in RRMM patients with 1-3 prior therapies. To align with regimens available in routine clinical practice, and thus the availability of corresponding real-world data, only proteasome inhibitor (PI)-/immunomodulatory drug-based regimens were included. Of note, due to the inherent difficulty of obtaining precise progression dates in claims-based outcomes research and in some retrospective chart reviews, time to next therapy (TTNT) could be used as proxy for progression-free survival (PFS).
Results
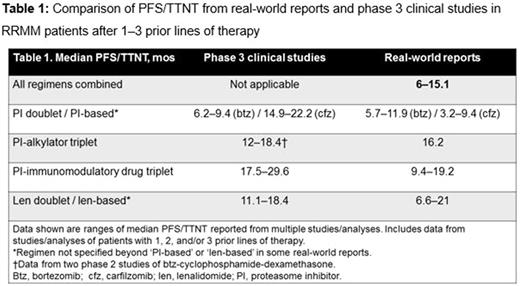
PFS/TTNT data from relevant real-world reports and from recent phase 3 clinical studies in RRMM are summarized in Table 1. The ranges of median PFS/TTNT values in the real-world reports were shorter than those reported in phase 3 clinical studies. Many real-world reports were population-based analyses encompassing a range of regimens in routine clinical practice, with other observational studies reporting outcomes for regimen-specific therapy, which may limit the indirect comparison of efficacy vs effectiveness outcomes.
Conclusions
The efficacy benefits reported in phase 3 clinical trials in RRMM do not translate equally into the real-world setting, with evidence of substantially shorter outcomes in the real-world vs clinical trial settings. Among other reasons, these disparities may be associated with discrepancies between enrolled populations in patient characteristics. Therefore, when interpreting results of clinical trials, it is critical to understand the context of the enrolled patient population and compare baseline patient characteristics. Clinical trial eligibility criteria typically exclude many patients from trial participation, and indeed up to 40% of MM patients enrolled in observational registries would not be eligible for interventional phase 3 studies (Shah et al, CLML 2017). Furthermore, there are differences in eligibility criteria among various clinical trials. Additionally, there is a discrepancy between duration of therapy achieved in the real-world vs clinical trial setting due to treatment center effect (academic vs community), study design (e.g. treatment to progression), and other factors. Therefore, there is an ongoing need to further understand the factors affecting the translation of clinical trial efficacy into real-world effectiveness in RRMM, and to design clinical trials more reflective of real-world scenarios.
Richardson: Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Research Funding; Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Research Funding. San Miguel: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees. Moreau: Onyx Pharmaceutical: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene, Janssen, Takeda, Novartis, Amgen, Roche: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Millennium: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Honoraria; Amgen: Honoraria. Hajek: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Pharma MAR: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria. Dimopoulos: Novartis: Consultancy, Honoraria; Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology: Consultancy, Honoraria, Other: Advisory Committee: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology; Genesis Pharma: Research Funding. Palumbo: Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Luptakova: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Skacel: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Kumar: Skyline: Honoraria; Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding. Anderson: C4 Therapeutics: Other: scientific founder; Oncopep: Other: scientific founder; MedImmune: Membership on an entity's Board of Directors or advisory committees; Millenium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.